OUR STRATEGIC APPROACH
The cornerstones of our strategy are the following:
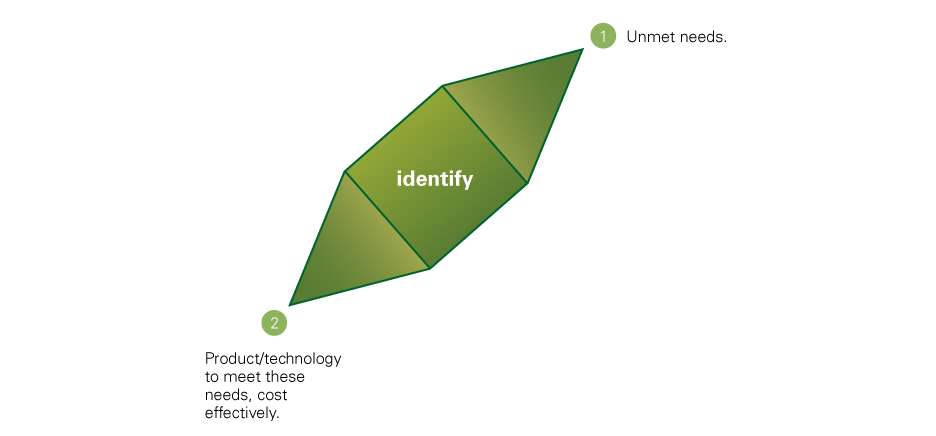
Innovation: Starting from a deep understanding of the patient journey, we identify still unmet medical needs in specialized therapeutic areas or the rare/orphan diseases field to be addressed through drug repurposing or the application of novel drug delivery technologies (preferably platform technologies protected by international patents), which are potentially applicable to several drug candidates in different therapeutic areas.
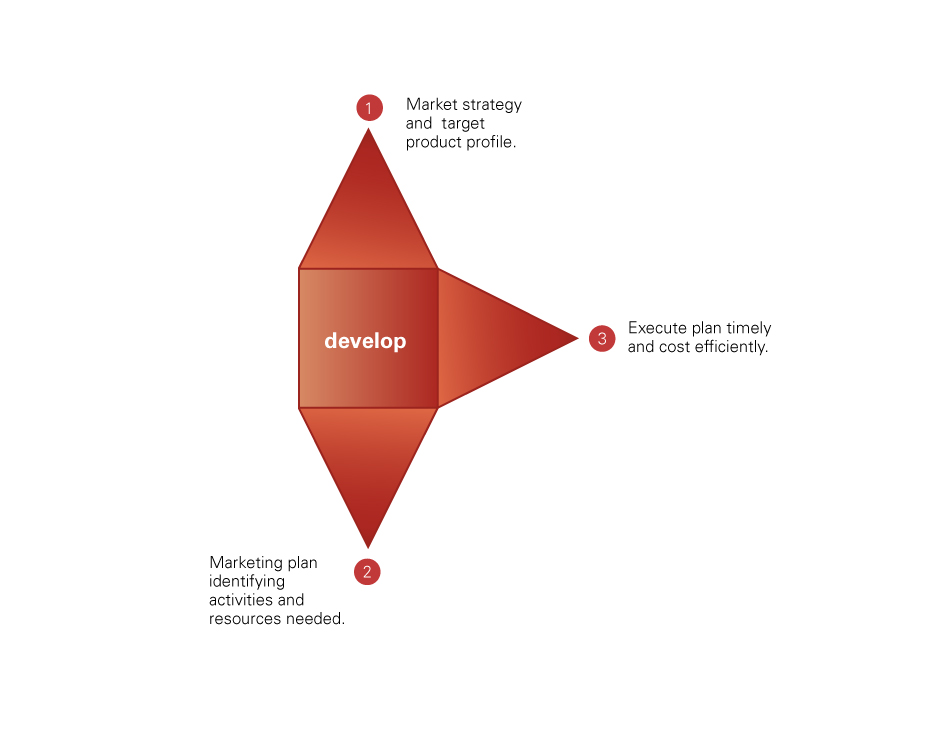
Development: Products are internally developed and financed (independently or with our co-development partners who are able to provide complementary know-how and competencies) by directly managing and controlling the key steps of the process - from proprietary technologies applications and/or novel dosage forms finalization to pre-clinical and clinical activities, market authorization and regulatory approval obtainment. In case of need, we enter into strategic outsourcing relationships to complete the development as well as to manufacture our product candidates in a cost-effective manner.
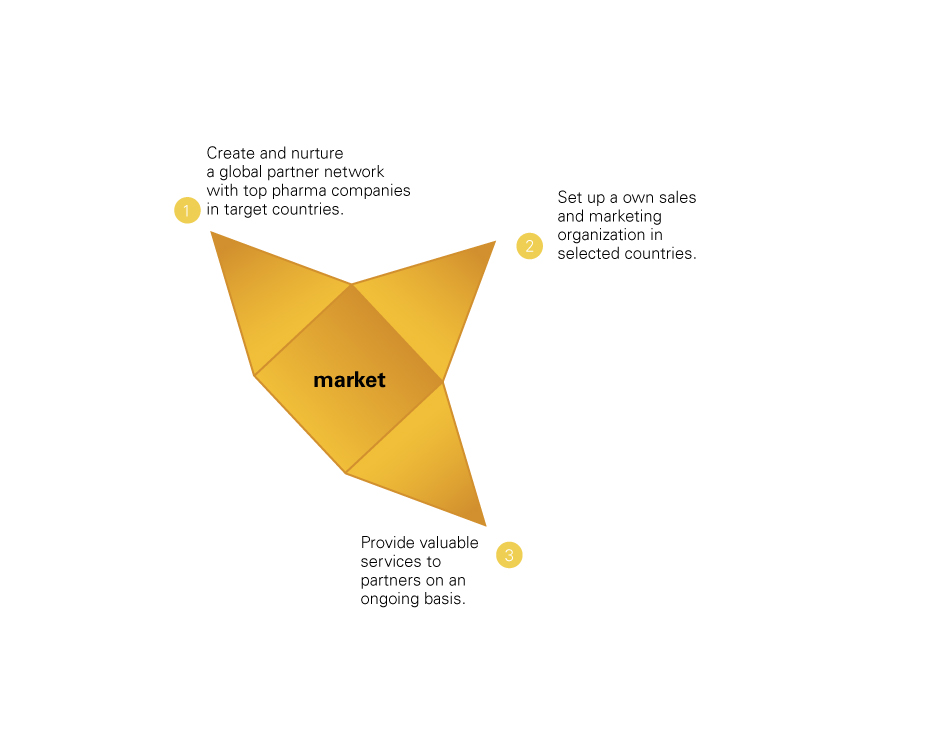
Partnering: We rely on solid companies for distribution, promotion, marketing and commercialization of our products on a worldwide basis.
Marketing: We promote and market selected products of our range in rare/orphan diseases with our own sales force and infrastructure in selected strategically significant countries.
Service: We support third party projects by offering pharma companies high-value contract development services based on advanced patented technologies, know-how and world-class pharma development expertise.