- About Us
- APR Products
- Our Services
- Media
- Contacts

Discover who we are and our partners global network, as well as learn more about our people.

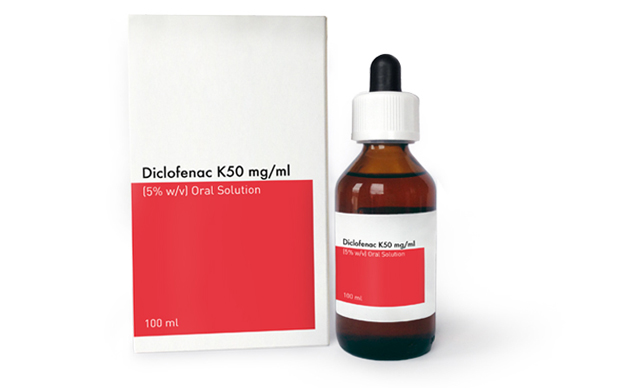
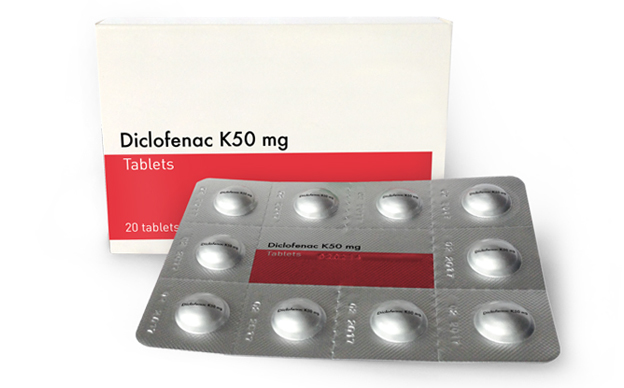
Learn more about our products and how they provide innovative solutions for patiens' unmet needs.

Discover our capabilities in project design services.

Find information such as website news, press releases and event presentations.

Get in touch with us to set up a meeting or propose your cooperation.